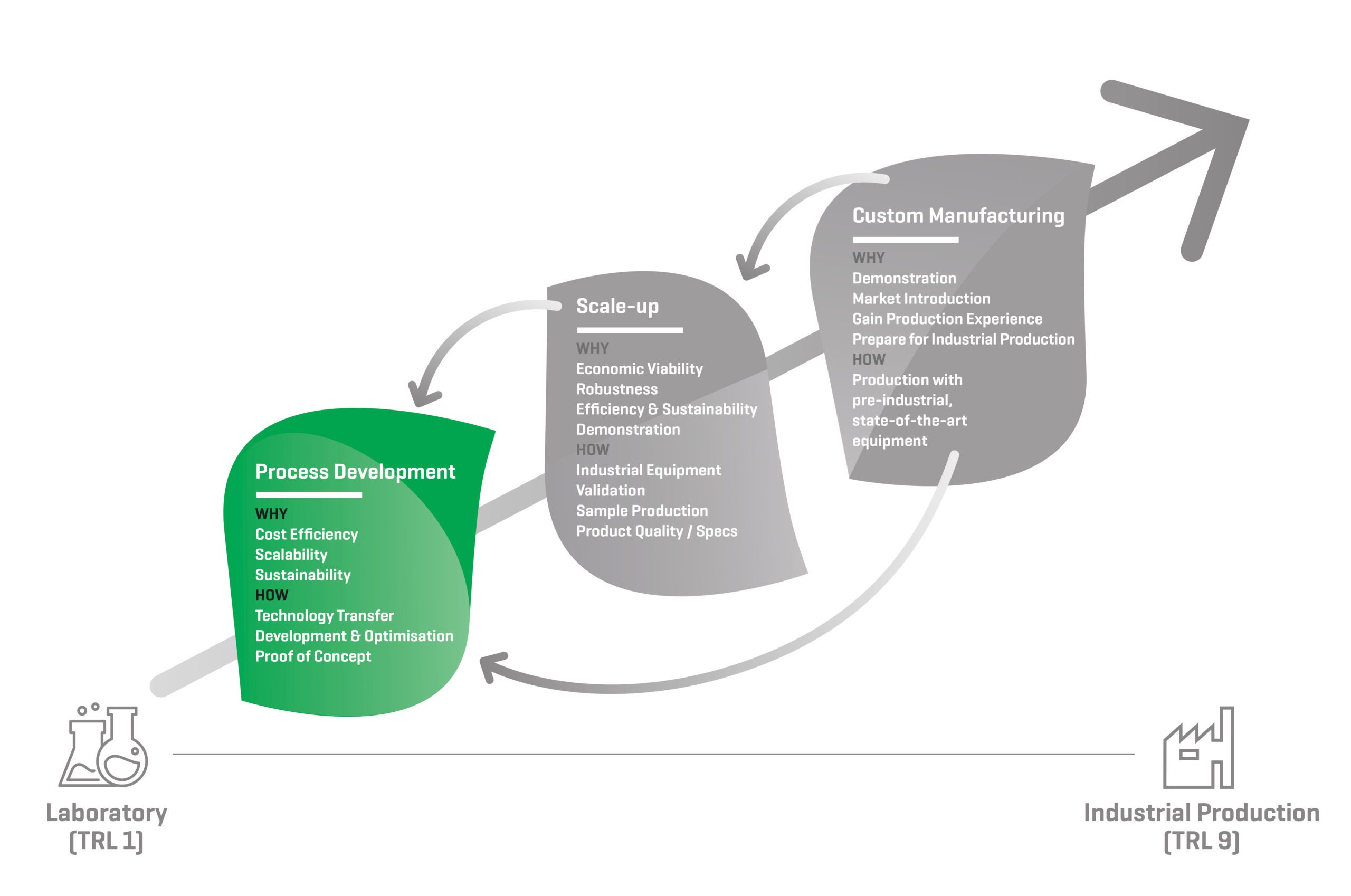
During the Process Development phase, the process is closely reviewed. The protocol is optimised to increase productivity, efficiency and sustainability, to allow scalability and to decrease the overall production cost. We prepare the process and product for scale-up and eventually for industrial application and commercialisation.
Process Development: Why?
COST EFFICIENCY
An essential objective of process development is to make the process economically viable. That means making a product that meets all quality expectations at minimal production cost. This involves definition of targets, and process development and optimisation until the targets and benchmarks are met.
SCALABILITY
Laboratory procedures, glassware and complex analytics are typically not applicable at an industrial scale. After receiving a laboratory protocol, we suggest scalable alternatives based on our extensive experience with industrial equipment. We test these scalable procedures during the process development phase, with our wide array of small pilot equipment. These small units are indicative for the industrial unit operations we operate at the 1 or 10 ton scale, which are again the smaller versions of the equipment found in full scale industrial facilities.
SUSTAINABILITY
A process can only be industrialized when it’s safe and sustainable for the production staff, equipment and environment. We assess the risks and safety of the use of chemicals before scaling-up a process. We check the compatibility of the ingredients and process conditions with the equipment, environment, applicable permits and legislations. During the process development phase we improve the sustainability of the process, e.g. by decreasing water usage, eliminating the use of organic solvents, replacing toxic or corrosive chemicals by safer alternatives, by converting a chemical step into an enzymatic one.
Process Development: How?
TECHNOLOGY TRANSFER
The first step of a development, scale-up or manufacturing project is the technology transfer from the customer’s labs to our plant. The tech transfer has multiple objectives: to reproduce the process at our facility, to train our personnel and become acquainted with the process and product, to implement and compare the analytics. Sometimes the outcome is substantially different, and we need to find out why. Keep in mind that a laboratory environment is a very “controlled” environment. Transferring a process successfully to another facility will also already indicate how robust the process is and which aspects need to be improved.
DEVELOPMENT & OPTIMISATION
During the process development phase we process simplicity and robustness by reducing ingredient cost, consumption of water, energy, materials, solvents and ingredients. We do this by testing different processing strategies, ingredients, materials and operating parameters, and by comparing alternative process routes.
For processes that are transferred to us at low technology readiness (TRL) levels, we perform fermentation development, i.e. screening of medium compositions, testing feed and aeration strategies, but also downstream process (DSP) development, i.e. testing different process routes and unit operations, or even in situ product recovery (ISPR). For biomass pretreatment, green chemical and biocatalytic processes, we apply the same development strategy and wide array of equipment, including pressure and ATEX reactors.
BBEPP uses an integrated approach in which development and optimisation can be performed on laboratory as well as small pilot scale, and can be combined with tech transfer and scale-up. Having each unit operation available at several scales allows us to compare and extrapolate process results and assess their impact on downstream steps. The strategy, steps and scales are adjusted to the specific objectives of each customer and project.
We increase the efficiency of a process by simplifying the process, by reducing the number of purification steps, by making the process more robust which reduces the failure rate and the need for close follow-up. At a pilot scale these aspects and mass balances are measured, cost drivers are identified and the most favorable route is selected for further optimisation.
Cost reduction also involves eliminating unit operations that require high investment costs, elaborate follow-up or high energy, water or chemicals consumption, and replacing them by robust, state-of-the-art equipment, or in some cases by units that are already available at the envisioned industrial site.
Since 2010, the Bio Base Europe Pilot Plant built up a vast expertise in the development of downstream processes (DSP). Many different purification technologies can be tested at four scales: from bench and 100 L small pilot scale, to 1000 L and 10000 L pre-industrial scales. Our equipment is listed in the “Product Recovery and Purification” folder, and we’re open for organizing trials with new equipment.
PROOF-OF-CONCEPT
After process development and selection of the most promising recipe and process route, the feasibility and scalability of the process is tested in small but representative equipment. This “proof of concept” reduces the risk that a unit operation will fail at a more expensive, larger scale.

 webdesign
webdesign