By scaling-up with a factor 10, 100, 1000 or even 10000, we transform your lab scale process into an industrially viable, robust and efficient biobased process up to 10 m³ or ton scale. We deliver high-quality, technical or food grade product samples for further evaluation such as formulation development, toxicity testing for regulatory purposes, application testing, evaluation of samples by customers. The steps and tests needed to achieve this transformation and scale-up factor depend on the complexity and sensitivity of the process.
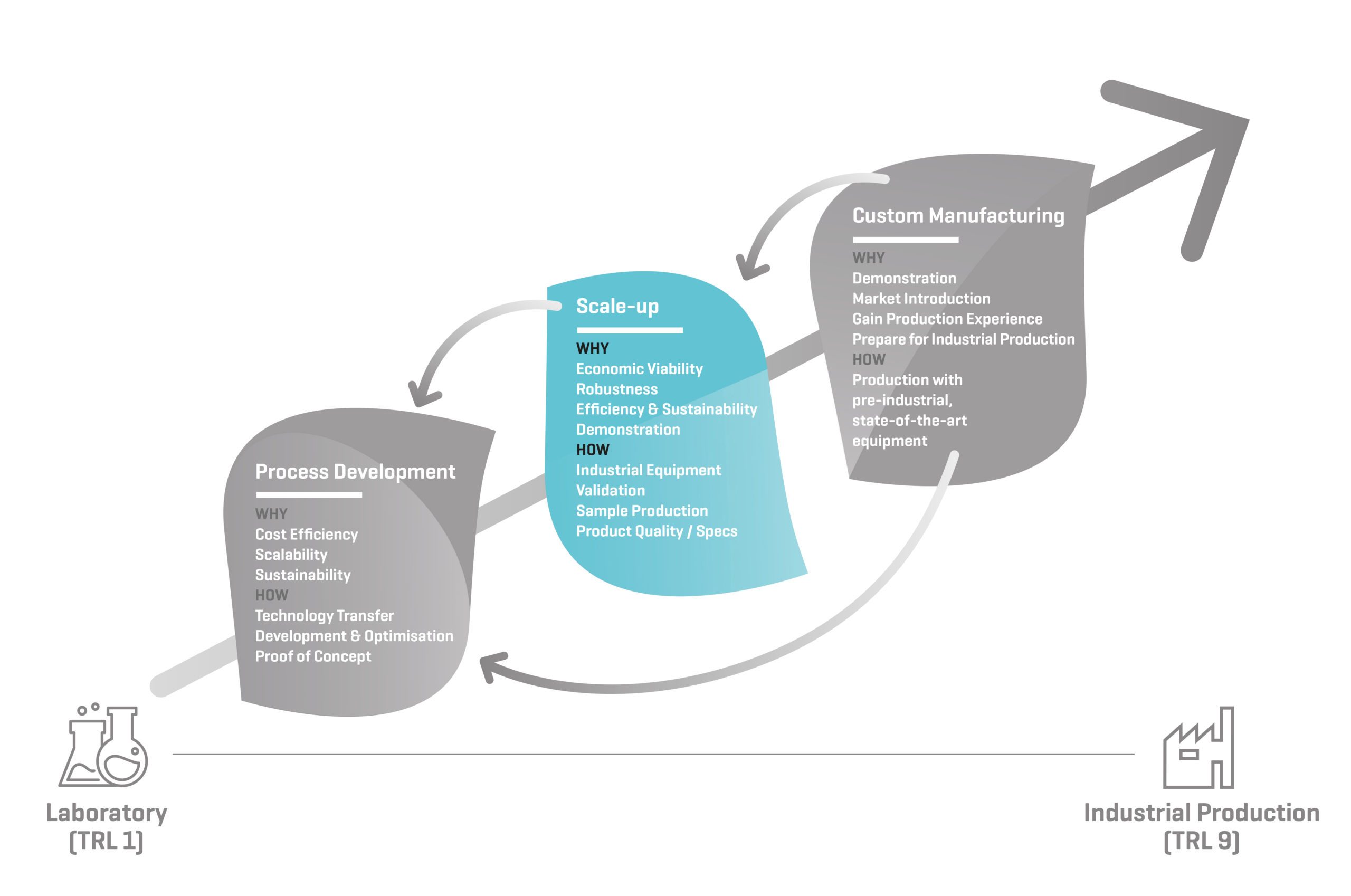
Scale-up: Why?
ECONOMIC VIABILITY
When scaling up a process by using (pre)-industrial equipment, the mass balances, final product quality, sensitive points of the process are closely monitored. This information will help to assess and improve the cost effectiveness of the process. How much raw materials, water and energy does your process use once it is running at (pre-) industrial scale? Do we need to re-think or re-engineer certain process steps or ingredients? The data generated during scale-up is reported in detail, so you can monitor the techno-economic viability of your process.
ROBUSTNESS
Assessing the robustness of the process once it is running on industrial equipment is critical. Knowing the reproducibility of your process and its response to process deviations/perturbations will allow you to better prepare for commercial production. A commercial environment typically doesn’t allow the same intensive follow-up and control as a bench scale process, and the process needs to be adapted for that. Reducing the failure rate is important to improve the economic viability of a process.
EFFICIENCY & SUSTAINABILITY
Biobased processes and products often replace fossil based counterparts. Our partners in consortium projects or customers perform Life Cycle Assessment (LCA) or Techno-Economic Analysis (TEA) to prove the environmental and economic sustainability based on the detailed mass balance and process data we provide of the scale-up trials.
Prior to scale-up trials we perform risk and compatibility assessments, to make sure the scale-up trials and later industrial production comply with safety standards for personnel, equipment, environment and final applications.
DEMONSTRATION
When the process steps and parameters are defined, the process can be run at the maximum scale and fixed settings to demonstrate the robustness and effectiveness of the process and to generate kilograms or tonnes of product for its final application. We build the process line and schedule the production to approximate the conditions of a dedicated plant. After successful demonstration, the process is ready for custom manufacturing at BBEPP or for transfer to a CMO or other industrial production facility. The data generated during demonstration is reported in detail and will facilitate tech transfer to a CMO or construction of a full scale facility by an engineering company.
Scale-up: How?
VALIDATION
We validate the developed production process at pilot scale using state-of-the-art industrial equipment. The equipment that is used in this stage is representative for the later demonstration scale, and for the ultimate industrial scale. This validation will allow prediction of capacities and workable process settings, and better scheduling of future larger pilot production campaigns.
SAMPLE PRODUCTION
We produce the first kilogram or tonne amounts of the innovative product which can be used for formulation and application testing, to convince customers or investors, to scout the market, or for quality control or regulatory approvals.
INDUSTRIAL EQUIPMENT
We use modular state-of-the-art industrial equipment from qualitative manufacturers for scale-up. We offer a wide array of alternative unit operations for all process steps even at different scales. Using our flexible units, instrumentation and tools, we efficiently build a customized process lines.
For more information: Download our Expertise Folders.
PRODUCT QUALITY and/or SPECIFICATIONS
During trials and production, the quality of intermediate and final products is monitored by online or laboratory analyses. (See our Analytical Capability and Cell Culture Lab Folder)
The Bio Base Europe Pilot Plant has a quality management system which is ISO9001 certified. We produce food grade products with an FSSC 22000 certificate, we are recognised as a “Belgian Food Operator”, we are registered with the US Food and Drug Administration. We can deliver religious certification upon request. For more information, take a look at our Quality and Food Safety Page.

 webdesign
webdesign